
Battery Care & Maintaining your Warranty
The information on the website is to help our customers get the maximum service life and performance from their batteries. The information provided will enable our customers get the most from their battery and keep it in optimum health. By keeping to the guide below, there can only be 3 ways your battery will fail, first is from old age, second is a manufacturing fault or lastly from physical damage.
The right care is essential to maximise the life of your Classic Car battery
Never leave your battery in a discharged state
A battery which has been fully charged can quite happily be stored away for 2-3 months. However, when a flat battery is stored for this period, you would almost certainly damage it beyond repair.
The reason for this is a chemical process called sulphation. When a battery is charged this chemical process cannot take place. However, when the battery’s voltage falls below 12.4 volts this process begins. The process causes sulphur crystals to form on the lead plates inside the battery, which in turn increases the battery’s electrical resistance. The longer this process is allowed to continue the worse the effect. Eventually the battery will become so electrically resistant, that you will be unable to charge the battery, let alone draw power from it.
If this process is caught early you may be able to salvage the battery using a battery charger with a pulse charge function. This will partially break down the sulphur crystals but the battery will never reach its full capacity again.
It is important to remember that if your battery fails due to sulphation this will not be covered under warranty. This kind of failure is classed as damage caused by the user through neglect.
Regularly checking the level of charge in your battery is good practice to ensure you achieve the best possible battery life.
Problems with lead-acid batteries: Sulphation and acid layering
If a battery is charged with a voltage which is too low, or if it always operates with a voltage which is too low (below 80%) acid layering, also referred to as stratification, occurs. The acid in the electrolyte stratifies due to poor mixing. Various densities cause layering of the sulphuric acid on the bottom and water in the top area of the battery. Because of this, only the middle section of the electrolyte, i.e. only a third, can be used for the discharging and charging process.
A possible cause of acid layering is mainly short journeys with the simultaneous use of a large number of electrical consumers. In this case, the alternator does not have enough time to recharge the battery.
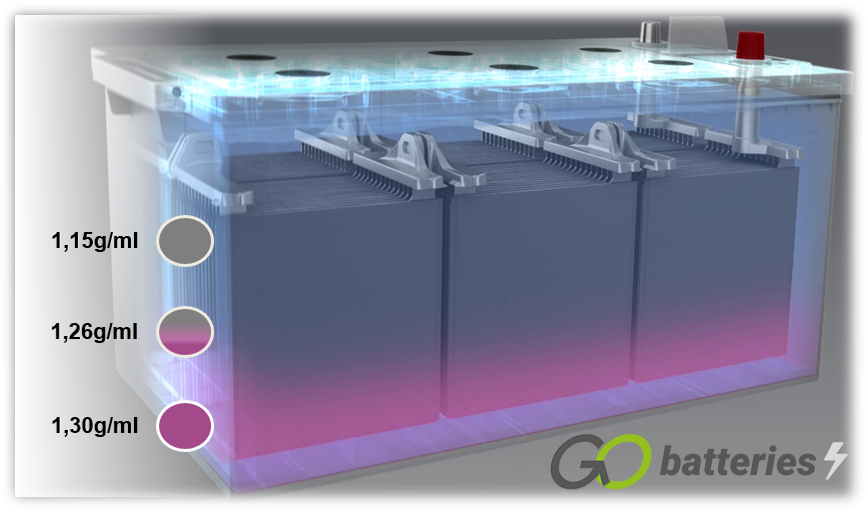
A result of acid layering is sulphation. If this occurs in the battery, or if it is not constantly charged to an adequate level, the lead sulphate (PbSO4) crystallises on the electrodes, to form larger crystal structures over the course of time. This process is known as “sulphation”. The crystallisation prevents the re-conversion of lead sulphate into the original components lead or lead oxide, which results in the prevention of charge acceptance and reduction in the cold start power.
Sharp crystals may also damage the separators or cause short circuits in the cells.
To counteract this effect and prevent premature battery failure, a battery should never be subjected to a low charge level over a long period. For this, it is advisable to test the battery regularly and to fully charge it if necessary.
Would you like to know more about this topic? How to properly charge a battery.
IMPORTANT – Never overcharge your battery
Although you must always keep your battery as charged as possible when not in use, you must never overcharge it. Overcharging will cause the battery to heat up and its electrolyte will start to evaporate. In turn this will cause the battery’s plates to break down, severely reducing the battery’s ability to yield power.
Overcharging can occur because of a faulty regulator on a vehicle’s charging system or by a manual charger being left on continuously at a high charging rate.
Fortunately, most chargers these days are now automatic and will turn themselves off once the battery has reached the end of a charging cycle.
This type of damage is also not covered under warranty, as the battery is clearly not at fault.
Avoiding deep discharge whenever possible
Everybody knows that all batteries will deteriorate over time, and will eventually have to be replaced. Every time you use your battery then recharge it, its performance is ever so slightly decreased. This cannot be avoided. However, the severity of this decrement can be limited.
The way to achieve this is to not discharge your battery too deeply. Deep discharging causes the performance decrement to be more severe. Therefore, once you have used the battery for the day, it is best to recharge rather than use it until it becomes flat.
Obviously, in the real world this is not always possible as the battery may be fully drained with one day’s use. But when you can, recharge the battery before it’s fully discharged.
Preparing your Battery
Before you plan on using your battery, you should complete the steps below to make sure it’s ready for use.
Charge level – Topping up if necessary
Regardless of what type of battery is used: you should always keep an eye on the charge level in order to maintain the highest possible charging capacity. Reliable and adequate charging of the battery can considerably extend its life. We know from above, you should have stored your battery in a charged state to avoid sulphation. However, even though the battery will be charged you should always perform a top up charge before touring. This will ensure the battery is in peak condition.

Checking the electrolyte levels in your battery
Most batteries these days are the sealed, maintenance-free type, but there are still a few open vent batteries on the market. If you have one of these you should always check the battery levels before touring. The level of the electrolyte should be just above the battery plates, ensuring the whole plate is submerged. Any part of the plate, which is not submerged, is prone to break down. This in turn will decrease the performance of the battery.
If you need to top up the battery levels, make sure you only use de-ionised water. Using tap water will cause mineral build up on the plates and reduce the performance of the battery.
For those of you with sealed, maintenance-free batteries this is not a concern, as they are designed to retain their electrolyte under normal conditions. The only way these batteries will have low electrolyte levels is if the battery is overcharged or a particular cell becomes faulty, causing overheating.

















